Doubling down on energy storage with metal-oxide magnesium battery
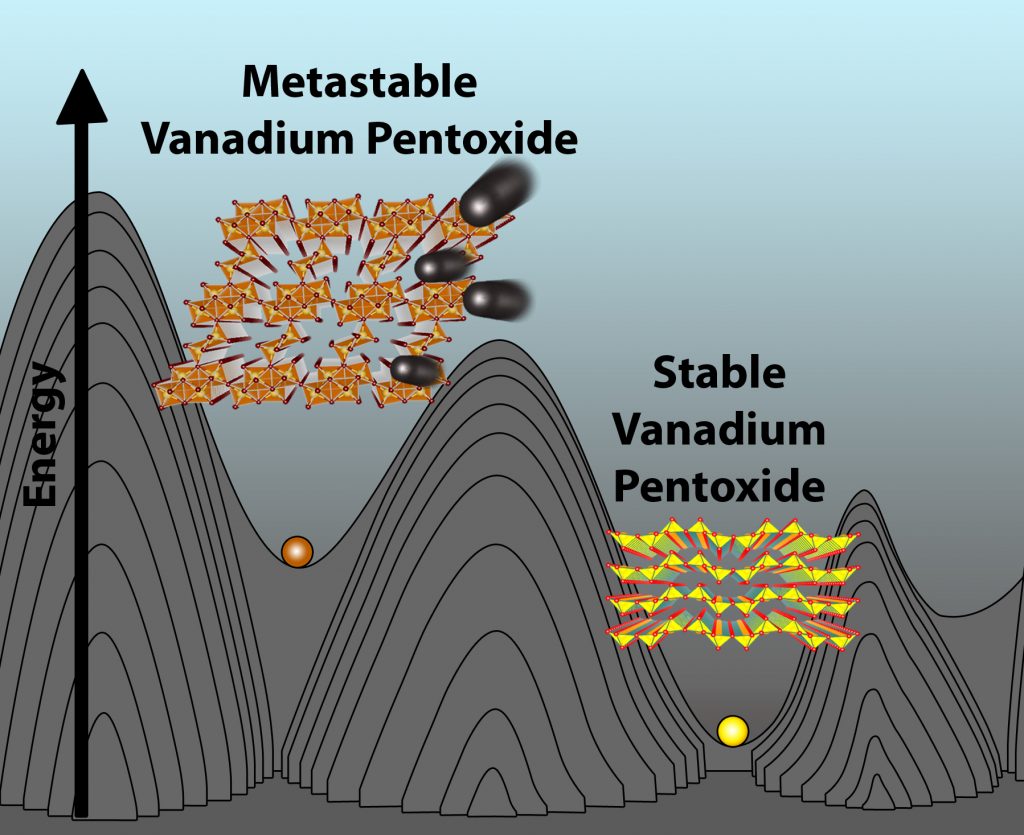
Image: College of Science
A multi-institution team of scientists led by Texas A&M University chemist Sarbajit Banerjee has discovered an exceptional metal-oxide magnesium battery cathode material, moving researchers one step closer to delivering batteries that promise higher density of energy storage on top of transformative advances in safety, cost and performance in comparison to their ubiquitous lithium-ion (Li-ion) counterparts.
“The worldwide push to advance renewable energy is limited by the availability of energy storage vectors,” says Banerjee in the team’s paper, published in the journal Chem, a new chemistry-focused journal by Cell Press. “Currently, lithium-ion technology dominates; however, the safety and long-term supply of lithium remain serious concerns. By contrast, magnesium is much more abundant than lithium, has a higher melting point, forms smooth surfaces when recharging, and has the potential to deliver more than a five-fold increase in energy density if an appropriate cathode can be identified.”
Ironically, the team’s futuristic solution hinges on a redesigned form of an old Li-ion cathode material, vanadium pentoxide, which they proved is capable of reversibly inserting magnesium ions.
“We’ve essentially reconfigured the atoms to provide a different pathway for magnesium ions to travel along, thereby obtaining a viable cathode material in which they can readily be inserted and extracted during discharging and charging of the battery,” Banerjee says.
This rare phenomenon is achieved by limiting the location of the magnesium ions to relatively uncomfortable atomic positions by design, based on the way the vanadium pentoxide is made – a property known as metastability. This metastability helps prevent the magnesium ions from getting trapped within the material and promotes complete harvesting of their charge-storing capacity with negligible degradation of the material after many charge-recharge cycles.
Banerjee, a Davidson Professor of Science in the Texas A&M Department of Chemistry and an affiliated faculty member in the Department of Materials Science and Engineering, has been working for a number of years to better understand ion intercalation — the critical process by which ions like lithium and magnesium move in and out of other materials within intercalation batteries.
Using one of the world’s most powerful soft x-ray microscopes – the Scanning Transmission X-ray Microscope (STXM) and X-ray Emission beamlines — at the Canadian Light Source in tandem with one of the world’s highest resolution aberration-corrected transmission electron microscopes housed at the University of Illinois at Chicago (UIC), Banerjee and collaborators from the Lawrence Berkeley National Laboratory, the UIC and Argonne National Laboratory were able to observe the unique electronic properties of their novel vanadium pentoxide and directly prove magnesium-ion intercalation into the material. Collectively, the team applied decades of combined experience in materials science to explain the fundamental reasons why this new type of vanadium pentoxide is superior to the old version as well as to Li-ion batteries.
Laptops and cell phones are two examples of the many technologies enabled by the rapid development of the lithium-ion battery, which revolutionized energy storage capacity and rechargeability in comparison to its lead-acid and nickel-metal hydride predecessors. However, given the widespread use of lithium not only in portable electronic devices but increasingly in the much larger batteries required for electric vehicles and grid energy storage, lithium is expected to be in increasingly short supply in the long-term. Furthermore, Li-ion batteries are a risky game, as highlighted by recent widely publicized reports detailed in Scientific American, Reuters and Forbes, for example, in which Li-ion-powered devices have either caught fire or exploded as a result of the fundamental flammability and reactivity of lithium.
“Apart from being much safer for consumer applications, magnesium-ion technology is appealing fundamentally because each magnesium ion gives up two electrons per ion — twice the charge, whereas each lithium ion gives up only one,” says Texas A&M chemistry graduate student and NASA Space Technology Research Fellow Justin Andrews, first author on the team’s paper. “This means that, all other considerations aside, if you can store as much magnesium in a material as you can store lithium, you immediately almost double the capacity of the battery.”

