‘Notoriously difficult’: Researchers study function of amino-acid radicals
With new funding from the U.S. Department of Health and Human Services, Texas A&M AgriLife Research will study the function of amino-acid radicals, which are fundamental to both beneficial and harmful chemical reactions in living organisms.
A better understanding of these chemical reactions is likely to impact widespread disciplines from anticancer drug development to sustainable solar energy production to explaining how non-steroidal anti-inflammatory drugs, or NSAIDs, such as aspirin and ibuprofen function in the body. However, amino-acid radicals have been notoriously difficult to study.
“It is extremely challenging to experimentally resolve the thermodynamic and kinetic redox properties of a single amino-acid residue,” said Cecilia Tommos, professor in the Department of Biochemistry and Biophysics at Texas A&M University. “Additionally, amino-acid radicals are typically highly oxidizing and reactive species, which increases the barrier for experiential characterization even further.”
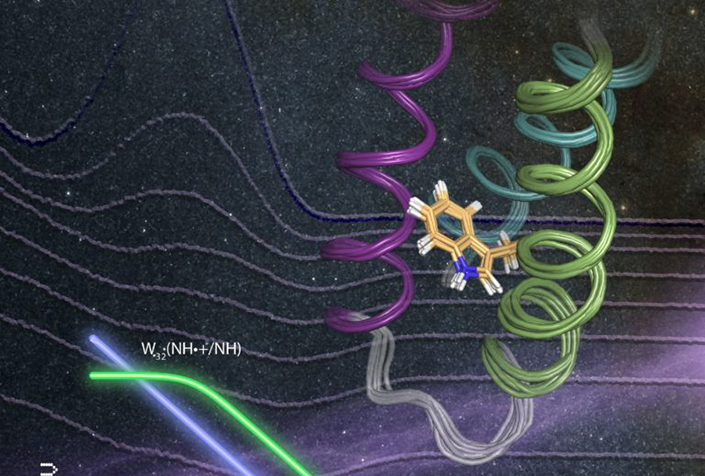
To address these challenges, Tommos’ research group has developed a library of well-structured model proteins that will enable detailed studies of the fundamental chemical properties associated with redox-active amino acids.
Tommos is the primary investigator for the study, which will be conducted in collaboration with other research groups at Yale University, California Institute of Technology and Uppsala University in Sweden. Funding of $303,000 annually will be provided for the four-year grant, making the total in excess of $1.2 million.
Enzymes that utilize amino-acid radicals are called redox proteins. These enzymes are essential in the function of numerous metabolic pathways in plants, animals and various microorganisms, Tommos said. Chemically speaking, redox proteins and amino-acid radicals enable a wide range of biochemical processes including energy transformation, signal transduction and DNA replication and repair.
Redox proteins use metallocofactors, organic molecules and four types of amino acids — tyrosine, tryptophan, cysteine and glycine — to perform electron transfer, ET, and proton-coupled electron transfer, PCET, reactions.
“The four amino acids serve as essential one-electron, or radical, redox cofactors in biocatalytic and multistep ET/PCET processes — some of which are essential to life on earth,” Tommos explained.
However, she noted there is also a “sinister side” to these electron transfer reactions. When these reactions are induced by oxidative stress, they can cause significant cellular damage.
Tommos said the family of well-structured model proteins her lab developed are specifically designed to study tyrosine and tryptophan radical formation, transfer and decay.
“This model system allows us to study amino-acid radical reactions under fully reversible conditions inside a structurally well-determined protein environment,” Tommos said.

